 Introduction to
Naval Weapons Engineering
Introduction to
Naval Weapons EngineeringIntroduction to Electro-optical systems
The Electromagnetic Spectrum
The full range of the electromagnetic spectrum covers frequencies
from 1 to 1024 Hz. We have already discussed communications
systems which dealt with the audio band, from 20-20,000 Hz, as
well as the radio band, which covered from 30 (ELF) up to 3 x
1011 Hz(EHF). Next, we looked at radar systems which
typically operate in a narrow range of frequencies, about 1-10
GHz. The microwave band, which covers wavelengths from
1 to 300 mm (frequencies from 109 to 3 x 1011
Hz), includes typical radar frequencies.
Beyond the microwave band lies the electro-optical band, which
covers wavelengths from 1 mm to 10 nm). The portion of the electro-optical
spectrum with frequencies below the visible portion is known as
the infrared (IR) band, with wavelengths from 0.7 to 1000
mm. It is further sub-divided as
follows:
Near IR: 0.7 - 1.5 mm
Intermediate IR: 1.5 - 8 mm
Far IR: 8 - 100 mm
Extreme IR: 100 - 1000 mm.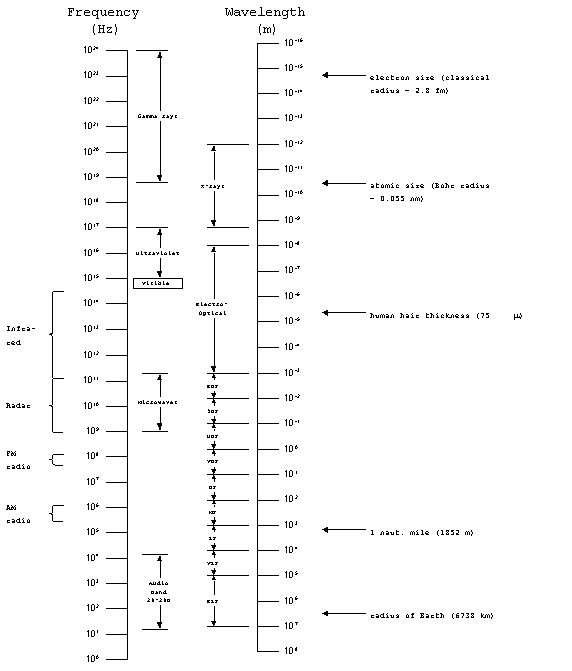
Figure
1. The electromagnetic spectrum.
The electro-optical band also includes the visible portion of
the spectrum which is approximately 0.4 to 0.7 mm
(1 mm, 10-6 m, is called
a micron, and is sometimes written as m
for a shorthand notation). In the visible portion, the wavelengths
are associated with the particular colors we see:
| Color | Wavelength range(mm) |
| Red | 0.63-0.75 |
| Orange | 0.59-0.62 |
| Yellow | 0.56-0.58 |
| Green | 0.50-0.55 |
| Blue | 0.43-0.49 |
| Violet | 0.37-0.42 |
Table 1. The visible spectrum.
Beyond the visible range, we have ultraviolet, x-rays and gamma
rays. At these high frequencies, the electromagnetic wave behave
less like waves and more like a highly energetic particle, called
the photon. The higher the frequency, the more energy
that is carried by each photon. High energy photons can be damaging
to materials and to humans. We are all familiar with the effects
of UV light, such as sunburn or eye irritation. At higher frequency
(and therefore energy), photons can damage materials or body tissue
and therefore are called ionizing radiation. In limited
amounts, X-rays can be used for imaging, while gamma rays are
most certainly deadly in large quantities.
Sources of Electro-Optical Radiation
Sources of electromagnetic energy can be divided into
two broad categories, selective and thermal radiators.
Selective radiators emit electro-optical radiation in a narrow
band about one or more particular frequencies. Therefore selective
radiators produce narrowband radiation. For example, the
He-Ne laser produces radiation in a narrow band about 0.6328 mm
(red light). Thermal radiators on the other hand, emit radiation
over a broad range of frequencies (or wavelengths). For example,
sunlight appears white because it has components covering all
frequencies in the visible spectrum and therefore appears as a
complete mixture of colors. Sunlight has components in the ultraviolet
and infrared as well.
Thermal Radiators
The electromagnetic energy that comes from any object
is known as thermal radiation. It is a physical property
of matter that all objects emit thermal radiation from their surfaces
in proportion to their absolute temperature. Absolute temperature
is defined in units of Kelvin (K), and may be found from the Celsius
scale by adding 273:
Tabsolute (Kelvin) = Tcelsius + 273.
For example, the freezing point of water, 00 Celsius,
is 273 K (note that the symbol for degrees is omitted when referring
to absolute temperature). The total power per unit area of thermal
radiation coming from an ideal object, called a black body,
at absolute temperature T can be predicted using the Stefan-Boltzmann
law:
M = sT4
where:
M = Power per unit area leaving the object. Also known as the
exitance, and has units of W/m2,
s = 5.67 x 10-8 W/(m2
K4). Called the Stefan-Boltzmann constant, and
T = the absolute temperature of the object.
The Stefan-Boltzmann law is based on the ideal source,
a black body. The idea of a black body is something that absorbs
all of the incident energy upon it. In order to maintain thermal
equilibrium, the object will radiate the same quantity of energy
away. It is called a black body, because we all know empirically
that black objects absorb more energy that others. Other objects
that reflect or transmit some of the incident energy are called
gray bodies. Since they absorb less, they radiate less.
The factor which describes both the amount of absorption and
also the radiation is called the emissivity, e.
It varies between 0 and 1, with 1 being the value of emissivity
for a black body. The exitance for a gray body is related to
the black-body by:
Mgray = eMblack
For objects that are opaque, the emissivity also describes how
much energy is reflected. The reflectance, r,
is defined by the relationship of energy conservation (which only
applies to opaque objects) that states:
e + r =1.
Below is a short table of values for common materials:
| material |
emissivity |
| metals | |
| Aluminum: polished anodized | 0.05 0.55 |
| Brass: rubbed with 80-grit emery heavily oxidized | 0.03 0.61 |
| Copper: polished heavily oxidized | 0.05 0 0.78 |
| Gold: polished | 0.02 |
| Iron: cast, polished cast, oxidized sheet, rusted | 0.21 0.64 0.69 |
| Magnesium: polished | 0.07 |
| Nickel: electoplate, polished oxidized | 0.05 0.37 |
| Silver: polished | 0.03 |
| Stainless steel (18-8) buffed oxidized | 0.16 0.85 |
| Steel: polished oxidized | 0.07 0.79 |
| Tin: plated sheet | 0.07 |
| others | |
| Brick | 0.93 |
| Carbon: candle soot graphite | 0.95 0.98 |
| Concrete |
0.92 |
| Glass: polished plate | 0.94 |
| Lacquer: white matte black | 0.92 0.97 |
| Oil: thick coating | 0.82 |
| Paint: oil-based | 0.94 |
| Paper | 0.93 |
| Plaster |
0.91 |
| Sand | 0.90 |
| Skin, human | 0.98 |
| Water: distilled ice snow | 0.95 0.96 0.98 |
| Wood: planed oak | 0.90 |
Table 2. Table of emissivities for common materials.
Example: thermal flux emitted by the human body.
The surface area of a typical person could be estimated at roughly
2 m2.
The body temperature is 98.6 0F = 37 0C
= 310 K.
The total flux, P = (2 m2)(0.98)(5.67 x 10-8 W/m2K4)(310
K)4
P = 1026 W.
This seems like a lot of power, which it is. In order for your
skin to remain at a nearly constant temperature, it must be in
equilibrium, meaning that the this outward flow of energy must
be matched by a similar amount of input. This comes from your
inside your body and from the absorption of radiation.
The net outward flow of energy could be computed by estimating
the inflow of energy as if the body was at the same temperature
as the environment, about 200C or 293 K.
The absorbed flux would be:
Pin = (2 m2)(0.98)(5.67 x 10-8 W/m2K4)(293
K)4
Pin = 819 W
Which gives a net outward flow of (1026 W - 819 W) = 207 W
This estimate seems high since this would require
207 J/s x 1 kcal/4184 J x 3600 s/hr x 24 hr/day
= 4275 kcal/day,
4000 kcal per day to maintain body temperature!
Here we have assumed that the surface was bare skin. In actuality
the emissivity should include the clothes we normally wear, closer
to 0.5, which would reduce the output to about 100 W or 2000 kcal/day,
which is more reasonable.
As we have said, this radiation will be distributed over
range of wavelengths. The wavelength corresponding to the peak
emitted power changes as a function of the temperature. The spectrum
also gets wider as the temperature increases. In the figure below,
these relationships are illustrated.
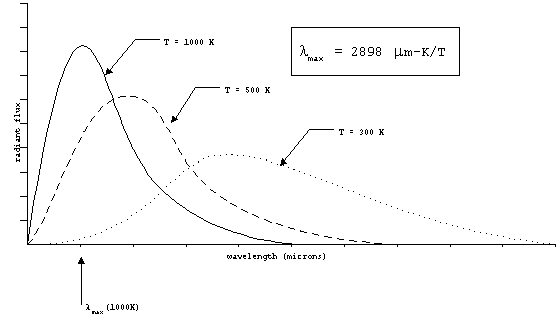
Figure 2. Wein's law.
lmax = 2898 mm-K/T
The wavelength of peak power can be predicted for a given target
temperature by Wien's law:
Note that the object temperature must be in Kelvin (K) and the
result is wavelength in microns. For the human body at 310 K,
the wavelength of peak thermal radiation is 9.3 mm.
The exhaust from a jet engine might be about 1000 K, with a peak
wavelength at 2.9 mm.
example: temperature of the surface of the Sun.
Measurements of the spectrum of sunlight show that lmax
= 0.55 mm. Using Wien's law in reverse,
the temperature of the surface of the Sun must be
T = 2898/0.55 = 5630 K (about 9700 0F).
Using this result, we can associate the different regions of the
infrared spectrum with characteristic temperatures:
Near IR (0.7 - 1.5 mm)
T = 1900 - 4000 K
Intermediate IR (1.5 - 8 mm)
T = 360 - 1900 K
Far IR (8 - 100 mm)
T = 30 - 360 K
So, we expect that personnel will be detectable in the far
infrared, while engines, machinery and jet exhaust will be
detectable in the intermediate infrared. Furthermore,
the background thermal radiation from the environment will only
affect systems that operate in near infrared.
Selective Radiators
Narrowband radiation originates when atoms or molecules
make transitions between discrete energy levels. For example,
the electron in a hydrogen atom can only have particular energy
levels. If the electron changes energy levels, the difference
in energy is radiated away as an electromagnetic wave. The set
of all possible transitions in a particular atom or molecule is
unique and can be used to identify the material. The study of
the spectrum of selective radiation is known as spectroscopy.
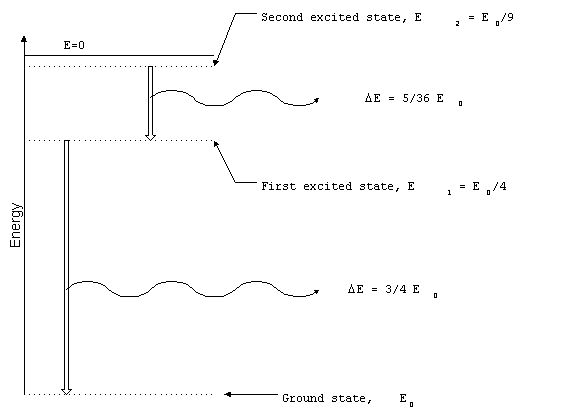
Figure 3. Transitions between
energy levels in hydrogen atom.
For weapons systems, the only selective radiator of interest is
laser light. The term laser is an acronym for "light
amplification by the stimulated emission of radiation." It
is a characteristic of most lasers to have an extremely narrow
bandwidth. A related property is called coherence, which
is the degree to which the light has a single frequency and phase.
Coherence can be either temporal, meaning the degree to
which the frequency and phase is stable in time, or spatial,
meaning the degree to which the frequency and phase at one location
matches that at another location. Temporal coherence is measured
by a coherence time, which is roughly the inverse of bandwidth.
Spatial coherence is measured by coherence length, which is nothing
more than the coherence time multiplied by the speed of light.
It is the great coherence length of typical lasers that allow
their beams to be narrowly focused.
Lasers may operate in a continuous wave mode, just like
CW radar, or in a pulsed mode. In a pulsed laser, the peak power
is related to the average power by the duty cycle (DC) which is
defined analogously to radar, DC = PW x PRF. Some typical lasers
are discussed below:
He-Ne. Helium and Neon gas mixture. Commonly used for laser
printing, interferometry and bar-code reading:
Gas ion. A class of noble-gas lasers including Argon, Krypton
and Xenon. Used in laser printers, medical treatment and as an
excitation source for other laser types.
Gas/Metal vapor. Uses a mixture of gas and metal vapor.
Common metal vapor laser uses Helium and Cadmium (He-Cd). Used
for fabrication and inspection of small objects like microchips.
Metal vapor. Uses pure metal gas like copper, gold or lead.
Used for high-speed photography, large-image projection television
and materials manufacturing.
CO2. Uses a mixture of carbon dioxide, helium
and neon. Operates in infrared region, often at high power, in
both CW and pulsed modes. Used for melting (CW mode) and cutting
or ablation (pulsed mode).
Excimer. Uses short-lived molecules combining rare-earth
atoms (such as Ar, Kr, and Xe) and halogens (Fl, Cl, Br, and I).
Commonly at ultraviolet wavelengths for materials processing (cutting
or ablation). Only in pulsed mode.
Chemical. Uses chemical compounds like HF or DF (deuterium
flouride) that are self-exciting, using the energy released from
the reaction. Used for weapons where large amounts of power may
not be available.
Free electron. These use a beam of electrons passed through
a periodic magnetic field. Tunable over a wide range of frequencies.
CW and pulsed modes. They have a high power capacity. Possible
use for weapons and materials processing.
Dye. Uses organic dye in liquid form. They form tunable
laser sources, within about 30-40 nm. There are many different
dyes used so the total range is 300-1200 nm. Used in research
applications where a specific wavelength is required.
Solid state. Common applications use yttrium aluminum garnate
(YAG) or glass doped with Nd, giving the Nd:YAG and Nd:Glass laser.
Other examples are the titanium sapphire (Ti:Al2O3).
Also used as an amplifying material for high gain laser systems.
Used for materials processing, surgery, range-finding and target
designation.
Diode. Uses combinations of semiconductor materials like
GaAs and AlGaAs. InP/InGaAs is also used. Used in electronic devices
(such as compact disk) and in fiber-optic systems.
| Type | Wavelength | Mode | Continuous Power | Peak Power |
| He-Ne | 632.8 nm | CW | 0.5-100 mW | - |
| Argon-ion |
514.5 nm | CW | 0.1-50 W | |
| He-Cd | 353.6 nm | CW | 10-200 mW | |
| CVL | 510.5 nm | Pulse | - | 1 MW |
| CO2 | 10.6 mm
| CW/Pulse |
1-10,000 W |
>1013 W |
| XeF | 351 nm | CW/Pulse | 100 W | >109 W |
| HF | 2.6-3.3 mm
| Pulse |
- | > 1 MW |
| Free electron | 8 mm - 248 nm | CW/Pulse |
10 W | >109 W |
| Dye | 300-1200 nm | CW/Pulse |
2 W | 109 W |
| Nd:YAG |
1.064 mm
| Pulse |
- | 1010 W |
| Diode | 0.5-1.55 mm
| CW | 1 mW - 5 W | - |
Table 3. Laser types.
Laser sources are divided into classes pertaining to the potential
for eye and skin damage:
Class 1: low power/non-hazardous
Class 2: low power/minor controls necessary. Only momentary
exposure is allowed.
Class 3: medium power/direct viewing hazard.
Class 4: high power/eye and skin hazard/ possible hazard of reflection
or fire hazard.