 Introduction to
Naval Weapons Engineering
Introduction to
Naval Weapons Engineering
Chemical Explosives

The main purpose of any warhead is to inflict damage on
the target. The way the damage is caused may vary with different
types of warheads, but in the most general sense, damage is caused
by the transfer of energy from the warhead to the target. The
energy is typically mechanical in nature and takes the form of
a shock wave or the kinetic energy of fragments. In either case,
a large amount of energy must be released. For many warheads
that energy is stored in the form of chemical explosives.
Explosive Reactions
There are many chemical reactions that will release energy.
These are known as exothermic reactions. If the reaction
proceeds slowly, the released energy will be dissipated and there
will be few noticeable effects other than an increase in temperature.
On the other hand, if the reaction proceeds very rapidly, then
the energy will not be dissipated. Thus, a great quantity of
energy can be deposited into a relatively small volume, then manifest
itself by a rapid expansion of hot gases, which in turn can create
a shock wave or propel fragments outwards at high speed. Chemical
explosions may be distinguished from other exothermic reactions
by the extreme rapidity of their reactions. In addition to the
violent release of energy, chemical explosions must provide a
means to transfer the energy into mechanical work. This is accomplished
by expanding product gases from the reaction. If no gases are
produced, then the energy will remain in the products as heat.
Most chemical explosions involve a limited set of simple
reactions, all of which involve oxidation (reaction with oxygen).
A relatively easy way to balance chemical explosive equations
is to assume that the following partial reactions take place to
their maximum extent (meaning one of the reactants is totally
consumed) and in order of precedence:
Table
1. Priorities of explosive reactions.
Priority
| Reaction (to completion)
|
1
| Metal + O Metallic Oxide (ex: ZnO or PbO)
|
2
| C + O CO (gas)
|
3
| 2H + O H2O (gas)
|
4
| CO + O CO2 (gas) (The CO comes from reaction (2))
|
5
| Excess O,H & N O2, N2, & H2 (gases)
|
Example- balance the combustion of TNT: C7H5N3O6.
No metals, so start with priority 2:
6C + 60 6CO, leaving 1C, 5H, 3N;
No oxygen left, skip priorities 3 and 4.
Lastly, the gases combine:
3N 3/2 N2
5H 5/2 H2, leaving 1 C not consumed.
Overall:
C7H5N3O6 6CO + 5/2 H2
+ 3/2 N2 + C.
The total amount of energy released in the reaction is
called the heat of explosion. It can be calculated by
comparing the heats of formation before and after the reaction
DE = DEf(reactants)
- DEf (products). The heats
of formation for the products and many common explosives (reactants)
are given in Table 2. The heat of explosion is defined so that
it will be positive for an exothermic reaction.
Table 2. Heats of formation.
Name
| Formula
| MW (g/mol)
| DEf (kJ/mol)
|
| CO
| 28
| -111.8
|
| CO2
| 44
| -393.5
|
| H2O
| 18
| -240.6
|
Nitroglycerin
| C3H5N3O9
| 227
| -333.66
|
RDX
| C3H6N6O6
| 222
| +83.82
|
HMX
| C4H8N8O8
| 296
| +104.77
|
PETN
| C5H8N4O12
| 316
| -514.63
|
TNT
| C7H5N3O6
| 227
| -54.39
|
TETRYL
| C7H5N5O8
| 287
| +38.91
|
Notes:
1) CO,CO2 and H2O are assumed
to be in gaseous form.
2) DEf for N2,H2,O2
and all other elements are all zero.
Example: find the heat of explosion for TNT.
Before: DEf = -54.4 kJ/mol
After: DEf = 6(-111.8)
+ 5/2(0) + 3/2(0) + 1(0) = -670.8 kJ/mol
DE = (-54.4) + 670.8 - = 616.4 kJ/mol,
Since DE > 0, the reaction is exothermic,
and the heat of explosion is +616.4 kJ/mol.
Expressed on a mass basis, TNT releases
kJ/mol)(1000 J/1 kJ)(1 mol/227 g) = 2175 J/g.
1 kg of TNT releases 2.175 x 106 J of energy.
Since most of the energy release comes from oxidation
reactions, the amount of oxygen available is a critical factor.
If there is insufficient oxygen to react with the available carbon
and hydrogen, the explosive is considered to be oxygen deficient.
The converse is considered oxygen rich. A quantitative measure
of this is called the oxygen balance, defined as:
OB = -(100 %)MW(O)/MW(explosive) [ 2C + H/2 + M - O]
where:
C,H,M&O are the number of moles of carbon, hydrogen, metal
and oxygen in the balanced reaction and MW is the molecular weight
of oxygen (= 16 g/mol) or the explosive.
Example- find the oxygen balance for TNT.
OB = -(100 %)(16/227)[2(7) + 5/2 - 6] = -72%
As a general rule, the oxygen balance should be near zero
to get the maximum amount of energy release. Other concerns like
stability or volatility often limit the oxygen balance for chemical
compounds. TNT is an example of a relatively powerful explosive
that is oxygen deficient.
Some explosives are mixtures of chemicals that do not react and
are known as composites. A common example is composite
B-3 which is made up of a 64/36 mixture of RDX (C3H6N6O6)
and TNT. If written in the same notation, it would be C6.851H8.750N7.650O9.300
and would have an oxygen balance, OB = -40.5%. ANFO which is
a 94/6 mixture of ammonium nitrate and fuel oil has a -0.6% oxygen
balance. Composite explosives generally have oxygen balances
that are closer to the ideal case of zero. Here are the mixtures
used for some common composite explosives:
Table 3. Composite explosives.
Name
| Composition
| Formula
|
AMATOL
| 80/20 Ammonium nitrate/TNT
| C0.62H4.44N2.26O3.53
|
ANFO
| 94/6 Ammonium nitrate/#2 Diesel oil
| C0.365H4.713N2.000O3.000
|
COMP A-3
| 91/9 RDX/WAX
| C1.87H3.74N2.46O2.46
|
COMP B-3
| 64/36 RDX/TNT
| C6.851H8.750 N7.650O9.300
|
COMP C-4
| 91/5.3/2.1/1.6 RDX/Di(2-ethyhexyl) sebacate/Polyisobutylene/Motor Oil
| C1.82H3.54N2.46O2.51
|
DYNAMITE
| 75/15/10 RDX/TNT/Plasticizers
| -
|
Strength of Explosives
The determining factor in the conversion of the heat of explosion
into mechanical work is the amount of product gases available
for expansion. In the case of TNT, 10 moles of gas are produced
for each mole of explosive. We can exploit this fact to make
predictions about the actual explosive strength of other chemicals.
This is known as the Berthelot approximation, which states
that the relative explosive strength of a material (as compared
to TNT on a mass basis) may be calculated on the basis of two
factors:
the change in internal energy (DE)
and
the amount of gas produced. If we combine these factors and put
in values for our reference, TNT, we obtain:
Relative Strength (%) = 840 Dn DE
/MW2
where:
Dn = the number of moles of gas per
mole of explosive
DE = the heat of explosion in kJ/mol
MW = molecular weight of explosive in g/mol
The factor of 840 accounts for the units and values of DE
and Dn for TNT.
Example- calculate the Berthelot relative strength for RDX
RDX: C3H6N6O6 3CO + 3H2O
+ 3N2
MW = 222 g/mol
Dn = 9 mol
DEf (before)= 83.82 kJ/mol
DEf (after) = 3(-111.8)
+ 3(-240.6) = -1057.2 kJ/mol
Therefore:
RS = 840 (9) (83.82 + 1057.2)/2222
RS = 175 %
The relative explosive strength calculated in this manner
is of limited use. What is really important is the actual strength
which can only be measured by experiment. There are a variety
of standard tests, most of which involve a direct measurement
of the work performed. Here are some example measurements for
RDX:
Ballistic mortar test: 140 %
Trauzl block test: 186 %
Sand crush test: 136 %
all of which compare favorably with our Berthelot approximation.
Categories of Explosives
Not only must explosive materials be highly energetic,
as characterized by the relative strength, but they must also
react violently. The speed of the reaction is vital to the build
up of a large amount of energy into a small volume. Reactions
that proceed slowly allow the energy that is released to be dissipated
(this is a consideration involving the interaction of the shock
wave with targets). An explosion will create either a shock wave,
throw fragments outward our both. If the energy release is slow,
the shock wave will be gradual and extended and the fragment velocity
low. On the other hand, a violent reaction will be characterized
by a very sharp (short duration, high pressure) shock wave and
large fragment velocities. This rapidity of reaction is called
the brisance, or shattering potential of the explosion.
It is a property of the material and the degree of confinement.
If an explosion is restrained initially, it can build up a large
pressure and achieve the same effect. The rapidity of the reaction
is used as a method of classification of explosive materials.
Explosive materials which react very violently (are brisant)
are known as high explosives. They are used solely for
their destructive power. In contrast, there are some materials
that react more slowly. These are known as low explosives.
They release a large amount of energy, but due to the relatively
slow rate of reaction the energy is more useful as a propellant
where the expansion of the gases is used to move projectiles.
An example would be gunpowder, which although quite energetic,
is classified as a low explosive and used primarily as a propellant.
It is true that confinement will increase the brisance of gunpowder
but there is a wide variety of materials that react much more
quickly and violently than gunpowder.
Initiation of the Explosive Reaction
Although the oxidation reactions that release energy in
explosive reactions are energetically possible, they do not occur
spontaneously. There is usually some small barrier that must
be overcome by the input of energy that will start the reaction,
which then will continue by itself until completion. The input
of energy to overcome the barrier is known as initiation (or detonation).
Sometimes only mechanical force is required like in the case
of nitroglycerin. In other situations, it requires heat like
from a match or electricity. The ease of which an explosive may
be detonated is its sensitivity. For safety considerations,
explosive materials are separated into three categories: those
which will detonate easily, called sensitive or primary explosives;
those which require slightly more energy to detonate, called intermediate
explosives; and those which require relatively more energy
to detonate, called insensitive or secondary explosives.
The terms refer to how the different materials will be physically
configured in a working explosive device.
Table 4. Common explosives and their uses.
Primary H.E.
(detonators)
| Intermediate H.E.
(boosters)
| Secondary H.E.
(main charges)
|
Mercury fulminate
| Tetrytol
| RDX
|
Lead azide
| PETN
| Comp-A,B,C
|
Lead styphnate
| Tetryl
| Cyclotol
|
Tetracene
| TNT
| HBX-1,3
|
DDNP
| | H-6
|
| | MINOL 2
|
| | Ammonium Picrate
|
Primary explosive materials are used to detonate the entire
explosive device. That is, they are usually connected to some
external device which starts the detonation. In this capacity,
the primary explosive is called the fuse. The energy from the
explosive detonation of the primary material is used to set off
the booster which in turn sets off the main charge which is made
up of secondary (insensitive material). This combination of a
small quantity of sensitive material used to set off a large amount
of secondary material is known as the explosive train. It is
called a train, because the events occur in sequence. The main
charge must be made up of insensitive material for the safety
of those handling the device. In practice, the fuse is rarely
stored with the device until it is required for use. In this
manner, the device remains relatively safe, since it is only made
up of secondary (insensitive) material and cannot be detonated.
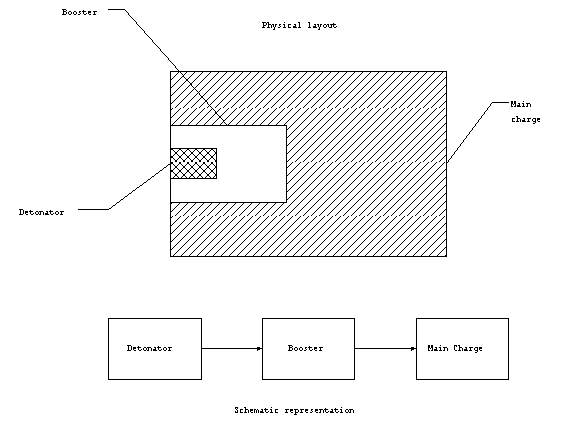
Figure 1. High explosive
train.
Once the fuse is installed, the entire device requires great care
in handling to prevent inadvertent detonation. Often, the device
is configured so that the explosive train must pass through a
small physical port that connects the fuse to the main charge.
This port can be blocked until the device will be used. As an
example, the port may consist of two rotating plates with off-center
holes. When the plates are aligned, the two holes will line up
and permit operation. This is called arming the device. Otherwise,
the holes will not be aligned and the device will be safe. The
mechanism with plates is called the safing and arming device.
Other configurations exist, but they all accomplish the same
function: to prevent inadvertent detonation and permit detonation
when authorized.

 Introduction to
Naval Weapons Engineering
Introduction to
Naval Weapons Engineering